Pirandamine
Nowadays, Pirandamine has become a very important issue in society. With the advancement of technology and globalization, Pirandamine has taken a fundamental role in our lives, influencing everything from our way of communicating to our political decisions. That is why it is crucial to thoroughly analyze the impact of Pirandamine on different aspects of our daily lives, as well as the challenges and opportunities it presents. In this article, we will explore the relevance of Pirandamine in today's world, offering a complete overview that seeks not only to inform, but also to generate reflection and debate on this significant topic.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
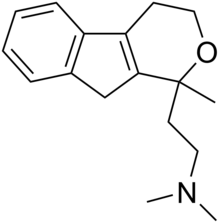
| Formula | C17H23NO |
| Molar mass | 257.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI). It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed. Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.
Synthesis

The Reformatsky reaction between 1-indanone (1) and ethyl bromoacetate in the presence of zinc gives ethyl 2-(1-hydroxy-2,3-dihydroinden-1-yl)acetate (2). The reduction of the ester with ester with LiAlH4 gives 1-(2-hydroxyethyl)-2,3-dihydroinden-1-ol, CID:130147665 (3). Acid catalyzed dehydration then leads to indene-3-ethanol (4'). Acid catalyzed condensation with ethyl acetoacetate then gives CID:53692067 (5) The saponification of the ester to the corresponding acid . The reaction of this with ethyl chloroformate would give a mixed anhydride, and further reaction of this with dimethylamine then led to the amide (6). Reduction with lithium aluminium hydride completes the synthesis of pirandamine (7).
See also
- Tandamine
- Prindamine (21489-22-5)
References
- ^ a b c Pugsley T, Lippmann W (May 1976). "Effects of tandamine and pirandamine, new potential antidepressants, on the brain uptake of norepinephrine and 5-hydroxytryptamine and related activities". Psychopharmacology. 47 (1): 33–41. doi:10.1007/BF00428698. PMID 1085452. S2CID 8354739.
- ^ Lippmann W, Pugsley TA (August 1976). "Pirandamine, a relatively selective 5-hydroxytryptamine uptake inhibitor". Pharmacological Research Communications. 8 (4): 387–405. doi:10.1016/0031-6989(76)90039-4. PMID 1088377.
- ^ a b Lippmann W, Seethaler K (April 1977). "Effects of tandamine and pirandamine, selective blockers of biogenic amine uptake mechanisms, on gastric acid secretion and ulcer formation in the rat". Life Sciences. 20 (8): 1393–400. doi:10.1016/0024-3205(77)90367-8. PMID 853871.
- ^ I. Jirkovsky, L. G. Humber and R. Noureldin,Eur. J. Med. Chem., 11, 571 (1976).
- ^ "Prindamine CAS#: 21489-22-5".