Diiminoisoindole
In today's article we are going to delve into the exciting world of Diiminoisoindole. From its origins to its relevance today, we will explore every relevant aspect of Diiminoisoindole in detail to provide you with a complete overview of this topic. Throughout the next few lines, we will discover the main key points, the latest trends and expert opinions on Diiminoisoindole. With this content, we hope to give you a deep and up-to-date understanding of Diiminoisoindole, so that you can enrich your knowledge and make more informed decisions on this topic. Get ready to immerse yourself in a fascinating universe full of nuances!
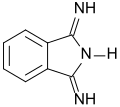 | |
| Names | |
|---|---|
| IUPAC name
3-Iminoisoindol-1-amine
| |
| Other names
Isoindoline-1,3-diimine; Phthalimide diimide[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.020.389 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H7N3 | |
| Molar mass | 145.165 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1,3-Diiminoisoindoline is a dye precursor used in industry.[2] The molecule can exist in different tautomers resulting in different crystalline solids.[3]
References
- ^ a b CID 18980 from PubChem
- ^ Venkataraman, K. (2 December 2012). The Chemistry of Synthetic Dyes. Elsevier. pp. 283–311. ISBN 9780323142953.
- ^ Zhang, Zhi-Qin; Njus, Jeffrey M.; Sandman, Daniel J.; Guo, Chengyun; Foxman, Bruce M.; Erk, Peter; van Gelder, Richard (2004). "Diiminoisoindoline: tautomerism, conformations, and polymorphism". Chemical Communications (7): 886–7. doi:10.1039/B400111G. PMID 15045113.